Abstract
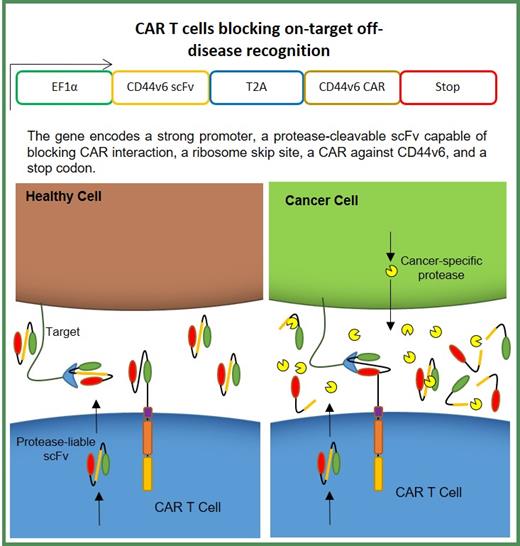
Adoptively transferred chimeric antigen receptor (CAR) T cells have shown significant promise in targeted immunotherapy against hematological tumors, despite concerns with safety and antigen escape. The CD44 adhesion molecule, which binds components of ECM such as collagen and hyaluronan, has been implicated in growth and survival as well as metastasis of tumor initiating stem cells (TSCs). A splice variant of CD44, CD44v6 is broadly expressed on malignant tumors including AML, CML and multiple myeloma (MM), where it aids tumor migration and predicts poor disease prognosis 1. Moreover, the expression of CD44v6 on healthy tissues including keratinocytes, presents a significant challenge in 'on-target, off-tumor' toxicity 2,3. We have developed a second generation, anti-CD44v6 CAR T cell (CD44v6CAR) that mediates potent cytotoxic and effector function against AML and MM in-vitro. Additionally, we observed lesser potency of our CD44v6CAR T cells against MM in comparison with AML in-vivo. Interestingly, higher levels of soluble CD44v6 antigen were also detected in mice engrafted with MM.1S compared to THP1. Possibly, the presence of soluble CD44v6 in mouse serum culminated in reduced anti-tumor activity against MM in-vivo, most likely due to CAR scFv blockade with antigen. Here, we provide an alternative strategy for improving the CD44v6 CAR T cell therapy and mitigating on-target off-tumor toxicity using CD44v6 CAR T cells that secrete a soluble protease-susceptible version of the CAR-expressed CD44v6 scFv that will block CAR binding in healthy tissue, but will be cleaved by cancer-specific proteases in the tumor site, allowing for CAR T cell binding and activation (Figure 1). We leveraged the presence of matrix metalloproteinase (MMP)-2, which is significantly overexpressed in multiple myeloma tumor microenvironment 4 to develop a modified CAR T construct (sCD44v6CAR) with engineered MMP-2 cut site in the linker between the heavy and light chains of the soluble CD44v6scFv. Kinetic analysis with varying MMP-2 concentration and digestion time showed moderate proteolytic susceptibility of our scFv construct, with digestion efficiency increasing in a dose-dependent manner. Furthermore, our engineered protease-liable scFv demonstrated higher affinity for CD44v6 antigen binding in titration assays compared to CAR scFv suggesting that engineered scFv are able to 1) bind and neutralize soluble antigen and 2) bind antigen on healthy tissues with high affinity to mitigate off-tumor toxicity. As expected, both conventional (CD44v6CAR) and modified sCD44v6 CAR T cells showed effective cytotoxicity against AML in vitro. Interestingly, cytotoxic activity against MM.1S using the modified T cells (sCD44v6CAR) was significantly suppressed, likely resulting from secretion of soluble scFv. The addition of recombinant MMP-2 in co-culture assays cleaved soluble scFvs, rescuing CAR-mediated tumor killing. Taken together, the data confirms our proof-of-concept hypothesis and highlights the protective capacity of engineered sCD44v6CAR T cells, with its ability to potentially neutralize off-target toxicity and improve anti-MM activity in future studies, which has impact on the CAR T cell therapy as a general strategy.
Figure 1: Schematic representation of CAR T Cell mitigating off-disease recognition. Presence of protease-susceptible linker in soluble scFv is efficiently cleaved by tumor-specific proteases in tumor microenvironment enabling CAR binding and activation. Lack of specific proteases in healthy tissues leads to high affinity, soluble scFv-target binding and effective blocking.
References
1. Heider, K. H., Kuthan, H., Stehle, G. & Munzert, G. CD44v6: a target for antibody-based cancer therapy. Cancer Immunol Immunother 53, 567-579, (2004).
2. Casucci, M. et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood 122, 3461-3472, (2013).
3. Riechelmann, H. et al. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral Oncology 44, 823-829, (2008).
4. Shay, G. et al. Selective inhibition of matrix metalloproteinase-2 in the multiple myeloma-bone microenvironment. Oncotarget 8, 41827-41840, (2017).
Forman: Allogene: Consultancy; Mustang Bio: Consultancy, Current holder of individual stocks in a privately-held company; Lixte Biotechnology: Consultancy, Current holder of individual stocks in a privately-held company. Wang: Pepromene Bio, Inc.: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal